Your Mouth’s Contribution to High Blood Pressure
- Kathleen Carson
- Jan 20
- 4 min read
The Nitric Oxide Pathway Disruption Model
Introduction: The Oral Origins of Nitric Oxide Disruption
January 2026 | By Dr. Kathleen Carson, DDS
Founder, Oral-Vitality
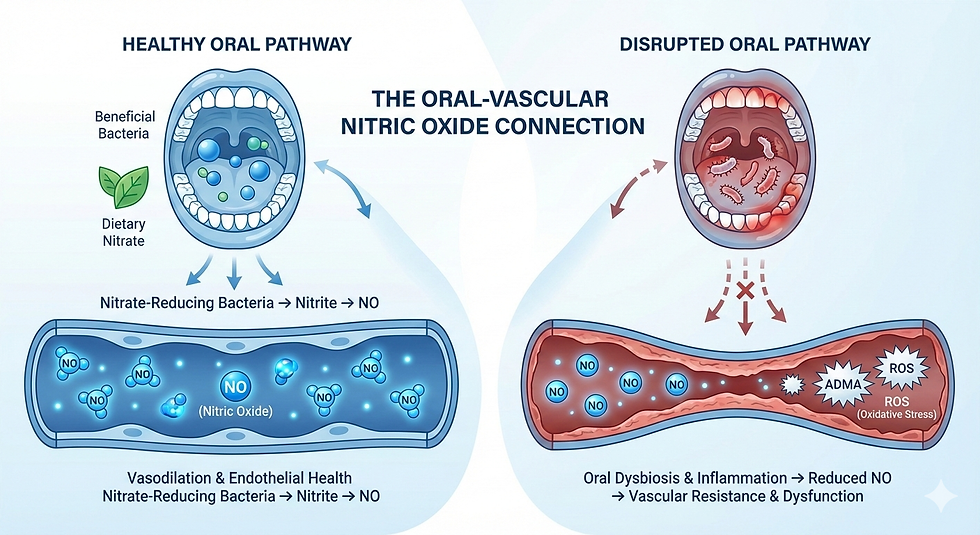
Emerging research suggests that the oral cavity plays a more influential role in vascular regulation than previously recognized. While hypertension has traditionally been attributed to renal, endocrine, genetic, and lifestyle factors, growing evidence indicates that oral dysbiosis and periodontal inflammation may influence nitric oxide (NO) bioavailability a central determinant of vascular tone and endothelial health.
This connection is mediated through several interrelated mechanisms, including disruption of nitrate-reducing oral bacteria, increased oxidative stress, and altered regulation of asymmetric dimethylarginine (ADMA), a well-established endogenous inhibitor of endothelial nitric oxide synthase (eNOS).Together, these processes support a biologically plausible model in which chronic oral inflammation contributes to impaired NO signaling, increased vascular resistance, and reduced endothelial adaptability.
Nitric Oxide as a Central Modulator of Vascular Function
Nitric oxide is fundamental to cardiovascular homeostasis. It regulates vasodilation, inhibits platelet aggregation, modulates arterial stiffness, and supports endothelial resilience. Impairment of NO signaling is a consistent feature of endothelial dysfunction and hypertension.
Physiologically, NO production is supported by two complementary pathways:
Endogenous pathway L-arginine → eNOS → nitric oxide
Enterosalivary nitrate pathway Dietary nitrate → oral nitrate-reducing bacteria → nitrite → nitric oxide
These pathways function in parallel and reinforce one another. Importantly, both are vulnerable to disruption in the setting of oral dysbiosis and chronic periodontal inflammation.
A substantial body of cardiovascular literature demonstrates that reduced NO bioavailability is associated with:
Increased vascular stiffness
Impaired endothelium-dependent vasodilation
Elevated central and peripheral blood pressure
Heightened oxidative burden
This framework positions the oral cavity as an underrecognized upstream site where NO regulation may be compromised.
Why This Matters Systemically: A Vascular Lens on Oral Dysbiosis
When oral microbiome balance is disrupted, nitrate-reducing species decline and periodontal pathogens proliferate. Two downstream systemic consequences may follow.
1. Reduced Nitric Oxide Availability
Loss of nitrate-reducing oral bacteria limits conversion of dietary nitrate to nitrite, reducing substrate availability for systemic NO production. The downstream sequence is mechanistically straightforward:
Reduced nitrate reduction → decreased nitrite → diminished NO → impaired vasodilation
This pathway provides a direct link between oral microbial composition and vascular tone.
2. Increased Oxidative and Inflammatory Load
Chronic periodontal inflammation generates a sustained inflammatory and oxidative environment characterized by:
Reactive oxygen species (ROS), including NOX2-mediated pathways
Pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α
Immune dysregulation extending beyond the periodontium
These factors directly injure endothelial cells, accelerate NO degradation, and promote eNOS uncoupling amplifying vascular strain. Together, they create a physiologic environment conducive to increased blood pressure and reduced vascular adaptability.
What the Evidence Shows: ADMA, eNOS Uncoupling, and Oxidative Injury
Multiple clinical and mechanistic studies support converging pathways linking periodontal inflammation with impaired vascular regulation.
1. ADMA Elevation as a Key Molecular Mediator
ADMA competitively inhibits eNOS, reducing endogenous NO production. Evidence suggests that:
Oxidative stress decreases activity of dimethylarginine dimethylaminohydrolase (DDAH), the enzyme responsible for ADMA clearance
Periodontitis is associated with elevated circulating ADMA levels
Higher ADMA concentrations correlate with impaired endothelial function and increased blood pressure
ADMA therefore represents a molecular bridge between oral inflammation, oxidative stress, and vascular dysfunction.
2. eNOS Uncoupling Under Oxidative Stress
In the presence of oxidative stress and cofactor depletion, eNOS may become “uncoupled,” shifting from NO production to superoxide generation. This transition:
Reduces bioavailable NO
Increases reactive oxygen species
Further destabilizes endothelial function
3. Peroxynitrite Formation
Nitric oxide readily reacts with superoxide to form peroxynitrite (ONOO⁻), a potent oxidant that:
Oxidizes lipids and proteins
Impairs endothelium-dependent vasodilation
Further reduces NO bioactivity
This process contributes to endothelial dysfunction commonly observed in hypertensive states.
4. Oral Microbiome Shifts Associated With Blood Pressure
Microbiome analyses demonstrate that individuals with elevated blood pressure often exhibit:
Reduced abundance of nitrate-reducing bacterial species
Increased prevalence of periodontal pathogens
Certain organisms, including Porphyromonas gingivalis, have demonstrated the capacity to directly impair endothelial function through adhesion, invasion, and toxin-mediated injury.
5. Periodontal Treatment and Vascular Markers
Although results vary across studies, some interventional trials suggest that periodontal therapy may be associated with:
Modest reductions in systolic and diastolic blood pressure
Improved flow-mediated dilation
Decreased systemic inflammatory markers
While these findings do not establish causation, they reinforce the biological plausibility of the oral-vascular connection.
Integration Within the Oral-Vitality Clinical Framework
The Oral-Vitality model evaluates oral findings through a systemic, mechanistic lens. In the context of blood pressure regulation, this includes:
Assessing oral microbiome balance, including nitrate-reducing capacity
Identifying inflammatory patterns associated with oxidative stress
Evaluating periodontal activity as a contributor to endothelial strain
Considering NO-related physiology during treatment planning
Coordinating with medical providers when cardiometabolic risk is present
Rather than positioning the mouth as an isolated site of disease, the Oral-Vitality approach interprets oral biology as a dynamic interface capable of influencing vascular physiology. This framework is designed to complement not replace standard cardiovascular evaluation and management.

Bottom Line
Periodontal inflammation and oral microbiome disruption may contribute to diminished nitric oxide availability, elevated ADMA levels, eNOS uncoupling, and increased oxidative stress all of which are associated with impaired endothelial function and higher blood pressure.
While oral factors are not the sole drivers of hypertension, they represent a potentially modifiable upstream component within a broader cardiometabolic landscape. Understanding and addressing these pathways may support endothelial health and contribute to a more comprehensive, physiology-informed approach to cardiometabolic risk management.





Comments